Personal, social, and environmental health
Adverse health outcomes can be driven by social and environmental determinants of health as well as individual behavior. Improving health outcomes requires addressing each of these factors. At the Hampton Roads Biomedical Research Consortium, we aim to improve health outcomes by leveraging domain expertise, incorporating community knowledge, and using machine learning to link and understand diverse multi-modal datasets.
Hydrodynamic models of social behavior
Community behavior arises from the decisions of each individual in the population. While physics provides tools to model large-scale behavior, incorporating individual preferences and decision-making remains an open problem. We use data-driven analysis to adapt hydrodynamic approaches to capture social behavior and examine a case study of residential dynamics in US Census data. Our model predicts an emergent societal memory at the transition between integration and segregation which slows community change. Collaboration with Daniel Seara, Michel Fruchart, Yael Avni, David Martin, and Vincenzo Vitelli (UChicago).
Predicting post-treatment lymphopenia in lung cancer patients
Circulating lymphocytes absorb significant radiation during treatment which can induce post-treatment complications such as a condition called lymphopenia. We developed an algorithm which estimates blood circulation through targeted organs, calculates the absorbed dose, and predicts lymphocyte death. Using this algorithm, we investigated treatment planning considerations for reducing lymphopenia in patients treated using stereotactic body radiation therapy. Collaboration with Wijesooriya group (UVA)Data-driven discovery in physics and biology
Physicists model the world around us by building predictive models often derived from fundamental principles like symmetries and conservation laws. Many soft materials and biophysical systems break these foundational assumptions, leading to behavior that can violate Newton's third law or detailed balance. This compleixty requires new approaches to model-building that can capture how, for example, cell motion might arise from protein interactions at the sub-cellular scale. We address this challenge and build interpretable models of diverse physical and biological systems by combining machine learning, physical theory, and close collaboration with experimentalists.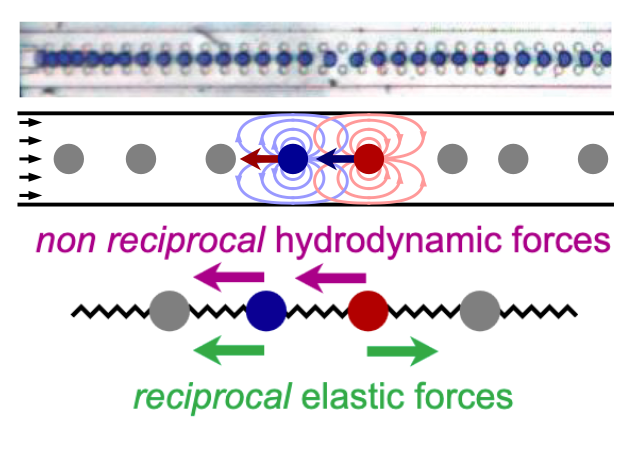
Nonlinear waves in non-reciprocal solids
We introduce a modeling pipeline that combines interpretable machine learning and continuum theory to guide discovery of new physics. Using a microfluidics experiment as a case study, we learn nonlinear dynamics directly from data using neural operators and sparse regression. We extract the governing equations and show how they originate from non-reciprocal hydrodynamic interactions between droplets. Collaboration with Alexis Poncet (ENS Lyon), Denis Bartolo (ENS Lyon), and Vincenzo Vitelli (UChicago).
Drosophila embryogenesis
Morphogenesis is how an organism gets its shape. In flies, this is regulated by genes which are tightly coupled to force-generating proteins throughout the embryo. We use machine learning to analyze movies of developing Drosophila embryos and discover equations governing the dynamics of morphogenesis. Using this model, we identify a genetic signaling pathway which sets the stage for embryo development. Collaboration with Michel Fruchart (ESPCI Gulliver), Vincenzo Vitelli (UChicago), and Streichan Lab (UCSB)